 Leading pharmaceutical companies including Johnson & Johnson, Bavarian Nordic, the Public Health Agency of Canada (PHAC) and GlaxoSmithKline (GSK) are the companies behind the impending Ebola vaccines trial in Ghana.
Leading pharmaceutical companies including Johnson & Johnson, Bavarian Nordic, the Public Health Agency of Canada (PHAC) and GlaxoSmithKline (GSK) are the companies behind the impending Ebola vaccines trial in Ghana.
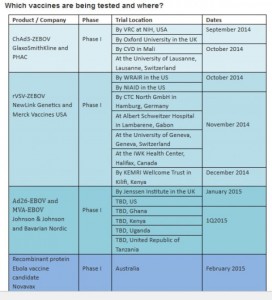
According to an official document on the World Health Organisation’s website, Phase I of the Ebola vaccines trial was expected to take place in the first quarter of 2015 in Ghana, Uganda, Kenya, USA and Tanzania.
A detailed breakdown revealed that Johnson and Johnson and Bavarian Nordic would take charge of the phase I of the trial while GSK and PHAC were in charge of the phase two.
Ghana’s Food and Drugs Authority (FDA) on Monday confirmed that vaccines for Ebola would be tested in Ghana, but dismissed reports that the trial will harm persons who will be used as ‘guinea pigs’ for the exercise.
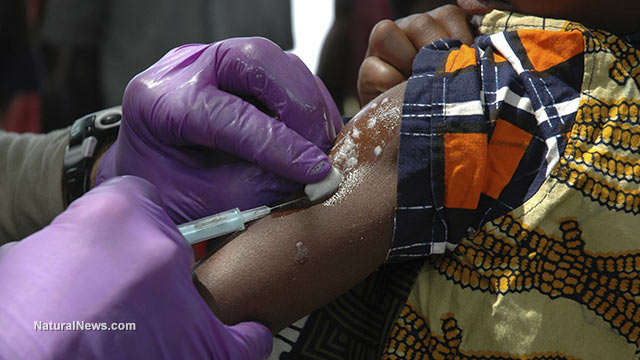
StarrFMonline.com revealed in May the planned vaccines experiment in Ghana, specifically at Hohoe in the Volta region of Ghana.
StarrFMonline.com sources revealed that the Hohoe Midwifery Training School has been selected for the project. As part of an enticement package, students who volunteer for the trial will be given GHc 200 and a cell phone as reward.
Several civil society organisations including the Ghana Academy of Arts and Sciences, Coalition for Ghana’s Independence Now (CGIN) and the Volta regional branch of the ruling NDC have all kicked against the exercise and called on government to stop the trial immediately.
The FDA has also explained that the vaccines that will be tested in Ghana do not treat Ebola Virus Disease but rather prevent it.
An Ebola vaccine test conducted in Kenya in March this year has shown it can protect people against the infection without causing side-effects.
Initial results from the Phase 1 trials of the candidate Ebola vaccine rVSV-ZEBOV released by the Kenya Medical Research Institute (Kemri) suggest that the vaccine is safe, and that it generates an immune response.
According to FDA documents, the vaccines to be tested in Ghana are made using a common cold virus called an adenovirus that “does not make people sick”.
“The vaccines contain extracts that do not cause the disease from the Ebola virus.
“It is expected that the body will recognise this extract as a foreign body and develop substances to fight it. These substances developed by the body against the extract may protect humans from the disease.
“In animals, such vaccines have been shown to stimulate immune response against the Ebola virus,” the FDA website said.